One question is always around us- what is dye? Is it colours or paints? In this article, we will try to solve your problems about the dye. That is Definition of dye, types of dyes, properties of dyes etc.
Definition of Dye-:
A dye is a coloured substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution and required a mordant to improve the fastness of the dye on the fibre. Dyes are substances capable of colouring of fabrics in such a manner that the colour cannot be removed by rubbing or washing. It should be noted that every coloured substance is not a dye. |
| Dye |
For example: Azobenzene is of orange red colour, but it is not dye, because it is not capable of colouring the fibre. Dyes can be used for imparting colour to the paper, plastic and leather.
What makes the dyes coloured?
Dyes are basically ionising and aromatic compounds, they have chromophores (an isolated function group capable of absorbing U.V. radiations e.g. -NO2, -N=O, -N=N-, -C=O, -C=C, -C=S etc. present in them. Their structure have aryl rings that has decolourised electron systems. These structures are said to be responsible for the absorption of electromagnetic radiation that has varying wavelengths, based upon the energy of the electron clouds.
Chromophores act by making energy changes in the delocalized electron cloud of dye. This alteration invariably results in the compound absorbing radiations within the visible range of colours and not outside it. Human eyes detect this absorption, and responds to the colour.
How can the colour of the Dyes be altered?
The colour of the dyes are altered by the Modifiers. The colour of methyl or ethyl groups are responsible for any alteration in the dyes, they alter the energy in the delocalized electrons.
Example:Methyl Violet series.
Auxochrome the only substance responsible for providing solubility and cohesiveness to dyes. An auxochrome is a group of atoms attached to a Chromophore, which modifies the ability of that chromophore to absorb light to a longer wavelength. Witt called these groups as auxochromes. Some of the auxochromes are -OH, -OCH3, -NH2 etc.
 |
Chromophore |
If chromogen has one or more auxochrome, the resulting substance is called a dye.
Dye= Chromogen + Auxochrome
Important functions of Auxochrome are:
They increase the intensity of colour.
e.g. 1) Nitrobenzene is pole yellow, as -NO2 group is a Chromophore.
2) p-nitroaniline is dark yellow, as -NO2 group is Chromophore and -NH2 group acts as an auxochrome.
3) Auxochromes make the chromogen a dye by fixing to the fibre due to the formation of a chemical bond between the fibre and auxochrome.
4) Thus, aniline yellow acts as a dye because it contains -N=N- (Chromophore) and -NH3Cl (auxochrome) which firms bond with fibre.
Auxochrome has the ability to intensify colours. Auxochromes are of two types, positively charged and negativity charged.
Quality of good dye:
All coloured substances are not dyes. However a coloured compound possessing following properties can be used as a dye.
1) It must have a suitable and attractive colour.
2) It must be able to attach itself to material from solution or to be capable of fixed on it.
3) Dye must be soluble in water or in a suitable solvent and should form a stable and good dispersion in water.
4) The substrate to be dyed must have a natural affinity for an appropriate dye and must be able to absorb it from solution or aqueous dispersion.
5) When a dye is fixed to a substrate, it must be fast to washing, dry cleaning, light, heat and other agencies. It must be resistant to chemicals like soap, washing soda, detergents, acids or alkalies.
There is probably no dye which can be guaranteed not to alter shade under all conditions.
Classification of Dyes:
Dyes can be classified in several ways, each class has a very unique chemistry, structure and particular way of bonding. Some dyes can react chemically with the substrates forming strong bonds in the process, and others can be held by physical forces.
Some of the prominent ways of classification are given below:
a) Natural /Synthetic
b) Organic/ Inorganic
c) By area and method of application
d) By nature of Electronic Excitation
e) Chemical classification : Based on the nature of their respective chromophores e.g. nitro, azo, xynthene, cyanine dyes.
f) According to the dyeing methods:
1) Anionic (for protein fibre)
2) Direct (cellulose)
3) Disperse (polyamide fibres).
Structures and Applications of dyes:
1) Nitro dyes:
A group of dyes, aromatic compounds whose colour results from the presence of a nitro (-NO2), hydroxy (-OH) and imino groups (-NHR; R= alkyl or aryl). They may also contain Cl, SO3H and COOH substituents.
Examples:
1) 2k disperse fast yellow is of practical importance for dyeing a number of fibres. It is produced by the reaction of 2,4-dinitro chloro benzene with p-amino phenol.
2) Naphthol yellow is used in a number of countries as a food dye. It is produced by Sulphonation of alpha-naphthol, with subsequent nitration of the product.
3) The barium salt of naphthol yellow is used for dyeing paper pulp and in the production of coloured pencils.
Nitro dyes were among the first industrial dyes. They lost their practical importance as a result of their low stability.
2) Nitroso Dyes:
 |
| Nitroso dye |
A group of dye; aromatic compounds containing a nitroso group(-N=O) in the ortho position to an hydroxyl group (-OH).
Example: 1-nitroso-2-naphthol.
Nitroso dyes are produced by the action of sodium nitrile on aromatic hydroxy compounds in acidic medium. Types B5 green mordant dye is the product of sodium bisulphite (NaHSO3) to 1-nitro-2-naphthol. The complex of this dye with divalent iron is a green pigment.
Applications of Nitroso dyes:
1) The green pigment is resistant to the action of heat and is commonly used for dyeing rubbers, in the production of wall paper and pencils, and in the paint and varnish industry.
2) The green pigment obtained directly on cotton fibre from B5 green mordent dye gives insufficiently stable colours.
3) Azo dyes:
These are organic dyes contain one or several azo groups (-N=N-) which connect aromatic radicals. Depending upon the number of such groups, the dyes are called mono-, di-, this, or polyazo dyes. Usually, azo dyes contain substituted or unsubstituted -NH2 and -OH groups in the aromatic nucleus and also NO2, Cl, SO3H, COOH and others. The percentage of acid groups ensures the water solubility of the dyes.
1) Acid dyes are used in dyeing silk and wool.
2) Direct azo dyes are used in colouring fabrics.
3) Active azo dyes give beautiful tints and fabrics and has resistance water and other processing agents.
4) Some azo dyes in a fine dispersion state are used in polygraphy and the paint and varnish industry.
5) Azo dyes are used mainly for colouring textiles but also for colouring leather, paper, rubber and certain palstics.
4) Heterocyclic dyes:
Organic compounds containing a chain composed of an odd number of methine groups, (=CH-) with conjugated double bonds. Some of the methine groups usually form heterocycles or aromatic residues. One of the simplest polymethine dyes used in photography has the structure-
Polymethine dyes: Cyanines are synthetic dyes belonging to polymethine group. Anthrocyanidins are natural plant pigments belonging to the polymethine dyes. These are fluoroscent dyes that may be attached to nucleic acid probes for different uses e.g. to accurately count reticulocytes.
 |
Heterocyclic dye |
Applications of Heterocyclic dyes:
1) Polymethine dyes are fast and have bright and rich colour.
2) They increase photosensitivity of silver bromide and are widely used in photography.
3) Many cationic polymethine dyes are also used in dyeing polyacrylonitriles fibres.
5) Phthalein dyes:
Phenolphthalein is a Colourless, odourless and tasteless crystalline compound that is only soluble in water but highly soluble in alcohol. The melting point is 259° -263° C. The compound is obtained through the condensation of phenol with phthalic anhydride.
1) It's 1% solution in alcohol is used as an indicator in the acid-alkali titrations.
2) In medicine, the compound is used as a laxative, the therapeutic effect of which derives from increased peristalsis of the large intestine.
3) The phenolphthalein tablet (0.1g) is prescribed as a treatment for chronic constipation.
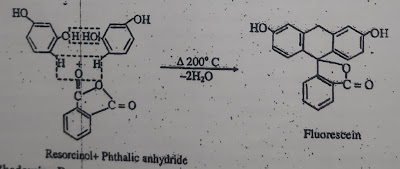
6) Xanthene dyes:
Xanthene is a yellow organic heterocyclic compound, soluble in diethyl ether. It's melting point is 101-192°C and it's boiling point is 310-312°C. Many xanthene dyes can be prepared by condensation of derivatives of phthalic anhydride with derivatives of resorcinol or 3-amino phenol.
Fluorescein is red powder. It is insoluble in water, but soluble in alkali to form a brownish red colour.
1) Xanthene are used chiefly in dyeing textile fibres, in colouring paper, in producing fluoroscent effects, and as organic pigments.
2) Fluorescein is used in tracing underground currents in sea and rivers as well as a marker during accidents.
3) The sodium salt of fluorescein is known as Uranine, which is used to dye wool, silk, in brilliant yellow from acidic bath.
7) Rhodamine dyes:
Rhodamine is a family of related chemical compounds, fluorone dyes, e.g.Rhodamine 6G and Rhodamine B. These dyes are generally toxic and are soluble in water Methanol and ethanol.
 |
Rhodamine dye |
Applications:
1) They are used as a dye and dye laser gain medium.
2) They are often used as a tracer dye within water to determine the rate and direction of flow and transport.
3) They are also used extensively in biotechnology applications such as fluoroscence microscopy, flow cytometry, fluorescence correlation spectroscopy.
4) Rhodamine 123 is also used in biochemistry to inhibit mitochondrion function and as a substrate of multidrug resistance associated protein.