Chemical Reaction
In simple word, chemical reaction is the reaction of two or more substances and produce a new substance.
Reactant:
The substance interacting during chemical reaction are called as Reactant.
Product:
The new substances are formed during chemical reaction is called as Products.
Sometimes the conversion of reactants into products is complete but sometimes the conversion is not complete no matter how long the reaction is allowed to continue. Thus is due to -
1) Reactants react to produce products this reaction called as forward reaction.
2) Products react to produce the reactants called as backward reaction.
1) Irreversible reaction:
A chemical reaction which occurs in only one direction i.e. forward direction and reactants are completely converted into products, called as irreversible reaction.
- Examples:
1) C(s) + O2(g) ------>CO2(g)
2) AgNO3(aq) +NaCl(aq) -----> AgCl(s) + NaNO3(aq)
3) 2Mg(s) +O2(g) ---->2MgO(s)
During above reaction, products do not react to produce back the reactants.
2) Reversible reaction:
A chemical reaction in which the reactants react to form product and the product once formed in turn converted back to the reactants is called as Reversible reaction.
Reversible reaction takes place simultaneously in both directions forward and reverse. Therefore this reactions are represented by the symbol (⇆).
Examples:
1) N2(g) + 3H2(g) ⇆ 2NH3(g)
2) 2SO2(g) + O2(g) ⇆ 2SO3(g)
3) H2(g) + I2(g) ⇆ 2HI(g)
Difference between Reversible reaction and Irreversible reaction.
During a chemical reaction, bonds in reactants are broken and such a process requires energy. Similarly new bonds are formed in formation of product and energy is released in this process.
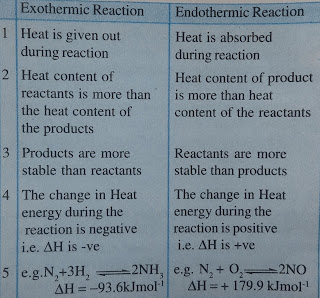
Endothermic reaction:
When the energy needed for breaking the bonds in the reactants is greater than energy released in new bond formation in the products, overall energy is absorbed by the reaction, is called as endothermic reactions.
A chemical reaction which proceeds with absorption of heat is called as endothermic reaction.
1) N2(g) + O2(g) ⇌ 2NO(g) ∆H= +79.9 KJ
2) H2(g) + I2(g) ⇌ 2HI(g) ∆H= +53.9 KJ
Here, to heat Content of the products is always more than that of total heat content of the reactants. Hence, the heat energy change is positive.
Exothermic reaction:
When the energy released in new bond formation in products is greater than that needed for breaking of bonds present in reactants, then overall energy is released by the reaction, is called as exothermic reaction.
In simple word, A chemical reaction which proceeds with evolution of heat is called exothermic reaction.
Examples:
1) N2(g) + 3H2 ⇆ 2NH3(g) + 93.6 KJ
2) NaOH(aq) + HCl (aq) ⇆ NaCl(aq) + H2O(l) + 57.3 KJ
Here, total heat content of the products is always less than the total heat content of the reactants. In this reaction, heat energy change is negative. i.e.
N2(g)+3H2(g)⇌2NH3(g) ∆H= -93.6 KJ/mol
Difference between Exothermic reactions and Endothermic reactions:
Rate of reaction:
Rate of reaction is defined as the decrease in concentration of reactant in unit time or increase in concentration of product in unit time.
In simple word, Rate of reaction is the change in concentration of reactants or products in unit time.
Factors affecting rate of reaction:
1) Chemical nature of reactants:
Rate of reaction depends upon the nature of bonds broken and formed during the reaction. When reactants are more reactive or less stable then rate of reaction is faster. And when reactants are less reactive or more stable then the rate of reaction is slower.
2) Size of particles of reactants:
Reactants which are in gaseous state or in form of miscible liquids or in dissolved form in a homogenous solution, rate of reaction is fast. The rate of reaction will be faster when solid is taken in powdered form than in lump form. Because of powdered form provides larger surface area than lump.
3) Concentration of reactants:
When the Concentration of the particles of reactants increases the rate of reaction also increases. Rate of reaction decreases with decrease in concentration of reactants. Thus the rate of reaction goes on decreasing with time.
4) Temperature:
Rate of reaction increases with increase in temperature for almost all reactions. i.e. rate of reaction is directly proportional to the temperature.
5) Catalyst:
Catalyst is a substance which is used to increase the rate of reaction without undergoing any change in its own mass and composition. i.e. Catalyst increases the rate of reaction.
Chemical Equilibrium:
Reversible reactions do not undergoes completion. The forward and reverse reactions are constantly converting reactants into products and products back into reactants. When the physical quantities including concentration of reactants and products remain constant with time at constant temperature is said to be equilibrium state.
A(g) + B(g) ⇌ C(g) + D(g)
The state at which concentration of reactants and the products remain constant under the given set of conditions is called as equilibrium state.
Initially the concentration of the reactants A and B are maximum but as the reaction proceeds, the concentration of reactants goes on decreasin
Finally a stage is reached at which the rate of forward reaction becomes equal to the rate of backward reaction. At this point the rates by which the reactants are converted into products and the products converted into reactant are equal.
Definition of equilibrium state:
The state in reversible reaction at which the rate of forward reaction is exactly equal to the rate of reverse reaction.
Temperature, pressure, concentration of reactants and products at equilibrium are called as equilibrium temperature, equilibrium pressure, and equilibrium concentration respectively.
Dynamic nature of equilibrium:
At equilibrium, under given conditions of temperature and pressure the reversible reaction appears to have stopped but in fact both the forward and reverse reactions are active and occur continuously with same rate. Thus, the equilibrium in reversible chemical reaction is dynamic in nature.
Characteristics of equilibrium state:
1. Equilibrium state is present only in reversible reactions not in irreversible reaction.
2. At equilibrium the rates of forward and reverse reactions are exactly equal.
3. All physical quantities of reaction remains constant at equilibrium. Examples: Concentration, Temperature, pressure, density, colour intensity etc.
4. The ratio of the product of the molar concentrations of products to the product of the molar concentrations of reactants of reversible reaction at equilibrium always remains constant.
5. Use of catalyst cannot change the position of equilibrium state.
6. Any change in equilibrium condition results in change of the position of equilibrium in a reaction as it affects the rate of forward or reverse reaction.
Law of mass action:
Law of mass action is stated by Guldberg and Waage.
It is relationship between the rate of reaction and molar concentration of reacting substances.
At a given temperature, the rate at which substance reacts is directly proportional to it's active mass and the rate of reaction is directly proportional to the product of active masses of reacting substances present at that instant.
K= [C][D] / [A][B]
Equilibrium constant:
It is the ratio of product of active masses of products to the product of the active masses of the reactants with each active mass term is raised to the power equal to the stoichiometric coefficient of the substance appearing in the balanced chemical reaction.
Kc= [C]^c [D]^d / [A]^a [B]^b
Where a, b, c and d are the stoichiometric coefficients of the substances A, B, C and D respectively.